42 label reactants and products
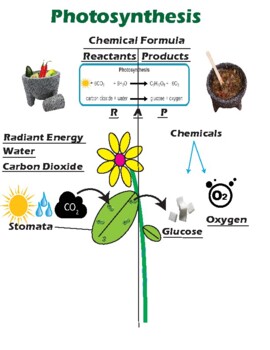
The Products & Reactants of a Chemical Reaction - Study.com The reactants and products of a chemical reaction can be identified by their position relative to the chemical reaction arrow : Reactants are always written on the left side of the arrow... Photosynthesis | Definition, Formula, Process, Diagram, Reactants ... photosynthesis, the process by which green plants and certain other organisms transform light energy into chemical energy. During photosynthesis in green plants, light energy is captured and used to convert water, carbon dioxide, and minerals into oxygen and energy-rich organic compounds. It would be impossible to overestimate the importance of photosynthesis in the maintenance of life on Earth.
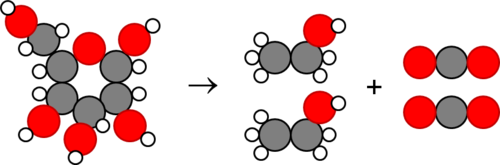
BISC104 - Chapter 2 Flash Cards Flashcards | Quizlet Label the reactants and the products of the following reaction. Some choices will be used more than once. 6 CO₂ + 6 H₂O -------> C₆H₁₂O₆ + 6 O₂ 6 CO₂ + 6 H₂O -------> C₆H₁₂O₆ + 6 O₂ reactant, reactant product, product In water, oxygen shares a bond with two hydrogen atoms.
Label reactants and products
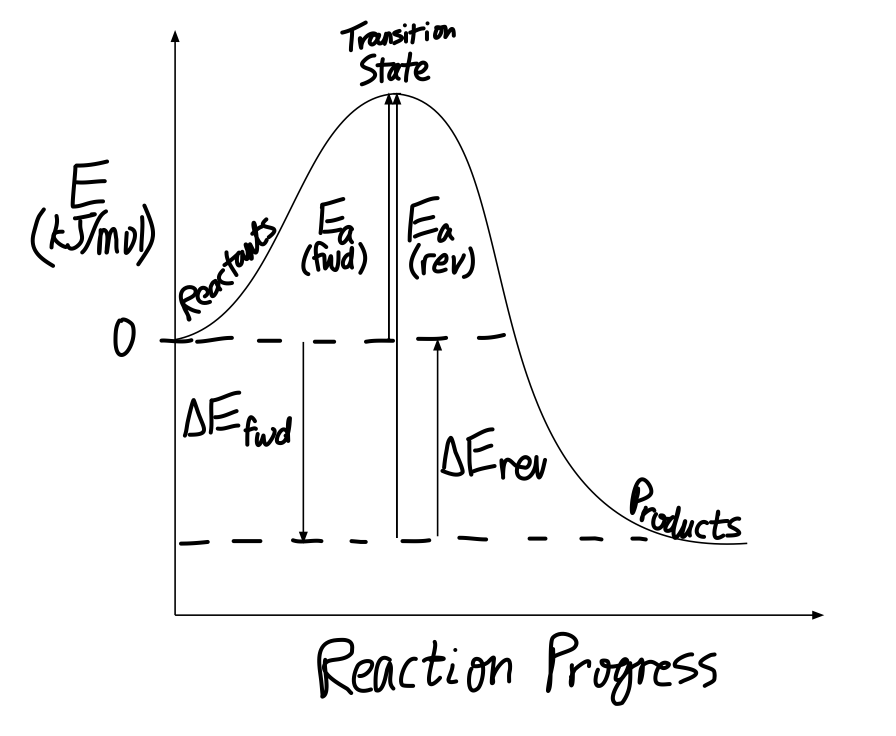
6.2.3.3: The Arrhenius Law - Activation Energies If the molecules in the reactants collide with enough kinetic energy and this energy is higher than the transition state energy, then the reaction occurs and products form. In other words, the higher the activation energy, the harder it is for a reaction to occur and vice versa. Effects of Enzymes on Activation Energy HW Solutions #9 - Chemistry LibreTexts Label the position of the reactants, products, and activated complex. Determine the heat of reaction, ΔH, (enthalpy change) for this reaction. ... Large energy barriers separates the reactants and the products so that only very energetic molecules can pass over this barrier. The forward reaction is exothermic. Double replacement reactions - Khan Academy The solvent for a double replacement reaction is usually water, and the reactants and products are usually ionic compounds—but they can also be acids or bases. Here is an example of a double replacement reaction: ... Neutralization reactions occur when the reactants are an acid and a base, and neutralization reactions are usually favorable as ...
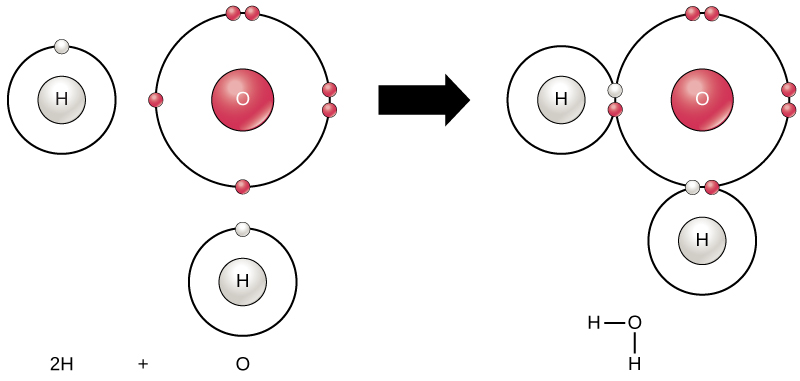
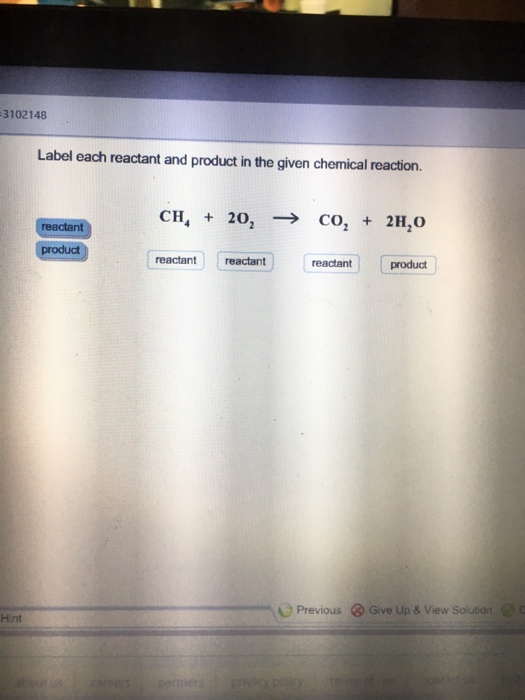
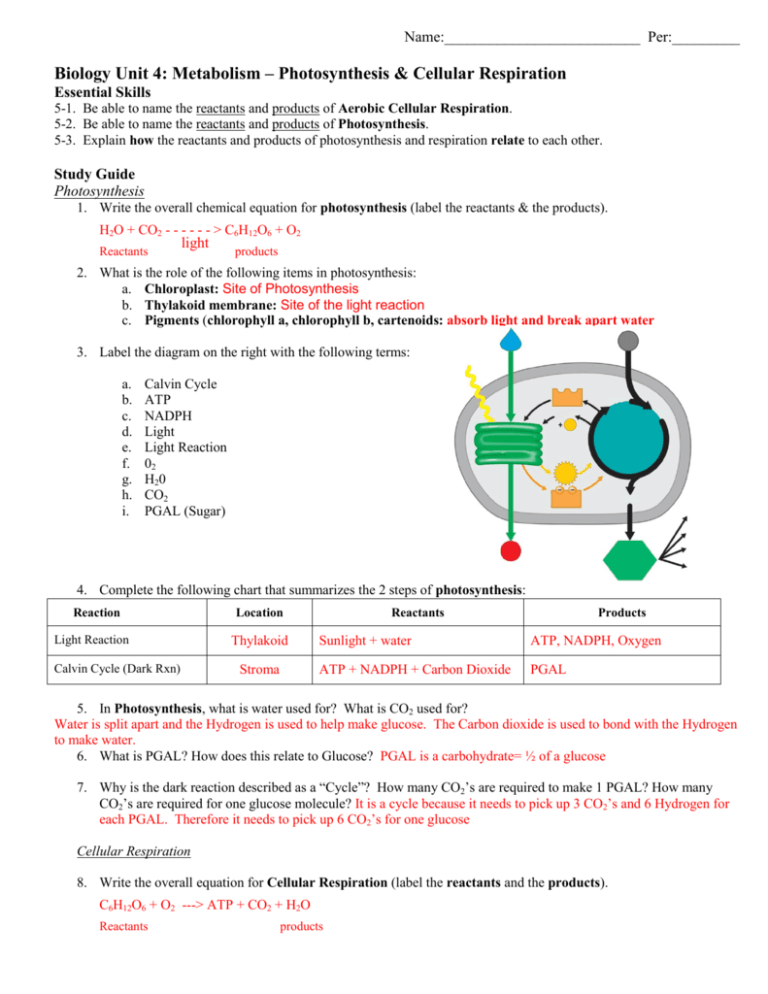
Label reactants and products. 2.17: Reactants and Products - Chemistry LibreTexts A reactant is a substance that is present at the start of a chemical reaction. The substance (s) to the right of the arrow are called products. A product is a substance that is present at the end of a chemical reaction. In the equation above, the zinc and sulfur are the reactants that chemically combine to form the zinc sulfide product. 7.3: The Chemical Equation - Chemistry LibreTexts Reactants and Products To describe a chemical reaction, we need to indicate what substances are present at the beginning and what substances are present at the end. The substances that are present at the beginning are called reactants and the substances present at the end are called products. 2.4 Flashcards | Quizlet Label the reactants and products in the chemical reaction: CH4+2O2----------------------> CO2+2H2O. Write brief definitions for these terms next to their labels. CH4+2O2- reactants: the substances changed during a chemical reaction; they are located on the left side of the equation Photosynthesis: Reactants and Products - Visible Body [Reactants] → [Products] You can think of the reactants as the ingredients for preparing a meal and the products as the different dishes in that meal. With that in mind, let's take a look at the chemical equation for photosynthesis: Sunlight + 6 CO 2 + 6 H 2 O → C 6 H 12 O 6 + 6 O 2 CO 2 = carbon dioxide H 2 O = water C 6 H 12 O 6 = glucose
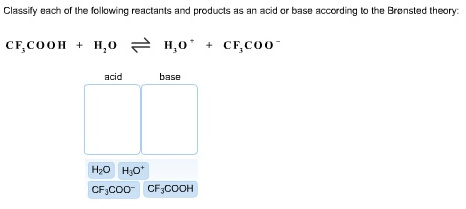
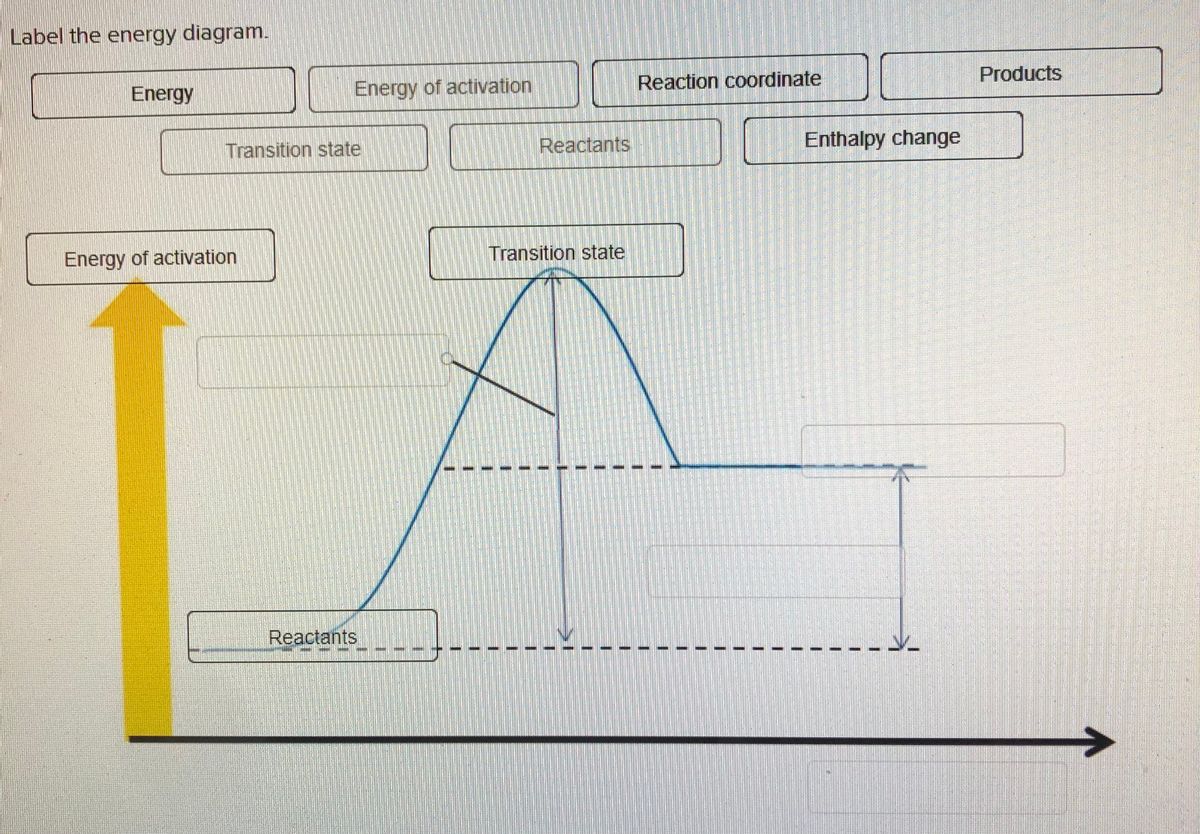
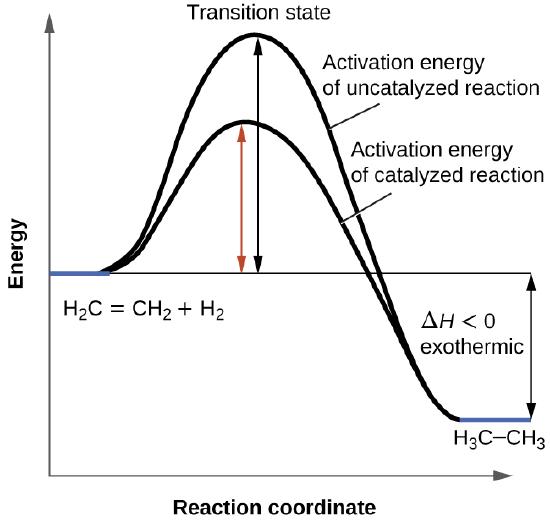
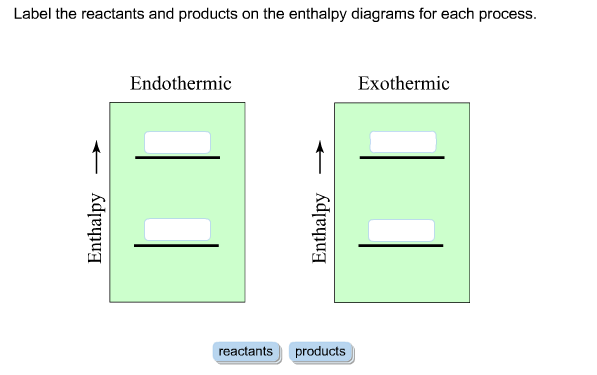
AP Chem - 6.2 Energy Diagrams of Reactions | Fiveable Introduction. Energy diagrams, also known as potential energy diagrams, can be used to represent the energy changes that occur during a chemical reaction. These diagrams show the potential energy of the reactants and products, as well as the activation energy required for the reaction to occur. The diagrams also indicate whether a reaction is ... Solved Label the reactants and products (for the forward - Chegg Label the reactants and products (for the forward reaction) given the following particulate model. A Conjugate acid BAcid C Base D Conjugate base NH3 (aq) + H2O (L) 근 NH4+ (aq) + OH- (aq) This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer Exothermic and endothermic reactions - AQA - BBC Bitesize The overall change in energy in a reaction is the difference between the energy of the reactants and products. Exothermic reactions The diagram shows a reaction profile for an exothermic... Energy Flow Concept 4 (Cellular Respiration) Flashcards Include which reactants and products (if any) are involved. Highlight what will be released as a product and what will move on to the next stage. Be sure to include where the process occurs in the Mitochondria. Glycolysis is the first stage in cellular respiration where glucose is broken down.
What Are the Reactants & Products in the Equation for ... - Sciencing The reactants for photosynthesis are light energy, water, carbon dioxide and chlorophyll, while the products are glucose (sugar), oxygen and water. Photosynthesis Reactants The photosynthetic process requires several simple reactants. Water is the first required reactant. The plant acquires water through its root system. Worked example: Calculating amounts of reactants and products The overall chemical equation says that 1 mole of glucose reacts with 6 moles of oxygen gas for the reaction to occur. So the glucose to oxygen ratio is 1:6, or basically we need 6 times as many moles of oxygen gas as we do glucose for the reaction to happen. So 0.129 x 6 = 0.833 moles of oxygen. Hope that helps. Endergonic vs exergonic reactions (article) - Khan Academy Exergonic reactions are also called spontaneous reactions, because they can occur without the addition of energy. Reactions with a positive ∆ G (∆ G > 0), on the other hand, require an input of energy and are called endergonic reactions. In this case, the products, or final state, have more free energy than the reactants, or initial state. Double replacement reactions - Khan Academy The solvent for a double replacement reaction is usually water, and the reactants and products are usually ionic compounds—but they can also be acids or bases. Here is an example of a double replacement reaction: ... Neutralization reactions occur when the reactants are an acid and a base, and neutralization reactions are usually favorable as ...
HW Solutions #9 - Chemistry LibreTexts Label the position of the reactants, products, and activated complex. Determine the heat of reaction, ΔH, (enthalpy change) for this reaction. ... Large energy barriers separates the reactants and the products so that only very energetic molecules can pass over this barrier. The forward reaction is exothermic.
6.2.3.3: The Arrhenius Law - Activation Energies If the molecules in the reactants collide with enough kinetic energy and this energy is higher than the transition state energy, then the reaction occurs and products form. In other words, the higher the activation energy, the harder it is for a reaction to occur and vice versa. Effects of Enzymes on Activation Energy



























Komentar
Posting Komentar